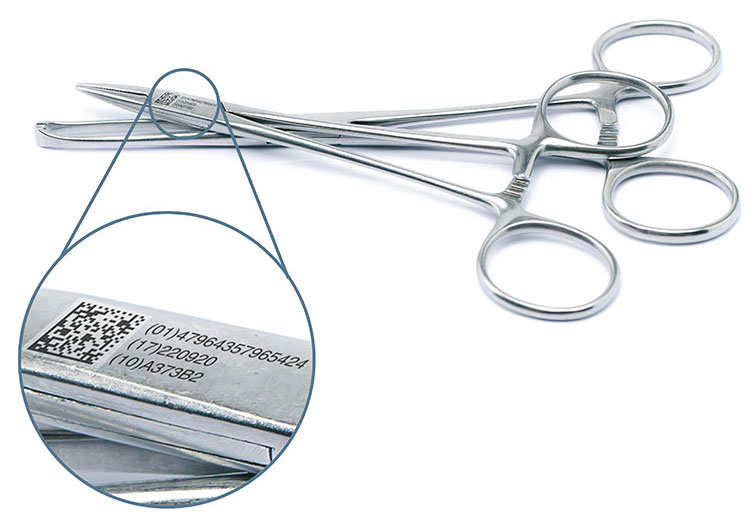
The European Medical Device Regulation (MDR): Coding requirements and deadlines at a glance
The impending deadlines of the Medical Devices Regulation (EU) 2017/745 will require manufacturers to apply specific codes called unique device identifiers (UDIs) to medical devices that are distributed in the EU.
Download our new infographic to learn more about UDI codes and the different risk classes of medical devices.